Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Postnatal Organellogenesis in Histaminergic Neurons of the Rat Brain
*Corresponding author:Ekaterina M Phedina, Department of Histology, Cytology and Embryology, Grodno State Medical University, Grodno, Bolshaja Troickaja str, 4, 230023, Republic of Belarus
Received: May 16, 2022; Published: May 24, 2022
DOI: 10.34297/AJBSR.2022.16.002218
Abstract
The organellogenesis of histaminergic neurons in the nucleus E2 of the rat hypothalamus on the 5th, 20th, and 45th days of postnatal development was described using the electron microscopic method of investigation. It has been established that during the described period of development in histaminergic neurons the number of mitochondria and the relative area occupied by them in the cytoplasm increase, the mitochondria acquire an elongated shape, and the length of the cristae increases in them. The number of bound ribosomes on the rough endoplasmic reticulum cisternae increases, while the number of free ribosomes does not change, the Golgi complex develops progressively, and the number of lysosomes and, accordingly, the relative area occupied by them in the cytoplasm also increase.
Keywords: Hypothalamus, Histaminergic Neurons, Cell Membrane, Nuclear Envelope, Organellogenesis, Lysosomes, Mitochondria, Golgi Complex, Autophagolysosome, Postnatal Period
Abbreviations: AL: Autophagolysosome; Aph: Autophagosome; Bm: Branched Mitochondria; CM: Cell Membrane; G: Golgi Complex; L: Lysosomes; LQ: Upper Limit of The Lower Quartile; M: Mitochondria; Me: Median; Mb: Multivesicular Body; NE: Nuclear Envelope; Np: Neuropil; N: Nucleus; RER: Rough Endoplasmic Reticulum; UQ: Lower Limit of The Upper Quartile
Introduction
The histaminergic neurotransmitter system, discovered much later than other aminergic systems of the brain, has attracted much attention from researchers in recent decades. It is a collection of histaminergic brain neurons and histamine receptors. In adult mammals and humans, the bodies of histaminergic neurons are in the tuberomammillary region of the hypothalamus, where they form five clusters - nuclei (E1-E5), which are spatially interconnected and gradually pass one into another [1,2]. The E2 nucleus is the largest: it makes up 40% of the total volume of the histaminergic nuclei and contains 54% of the histaminergic neurons of the hypothalamus [1]. Axons of histaminergic neurons spread to all parts of the brain, where they can coordinate the work of other neuronal systems [3- 5].
The literature describes spatiotemporal structuring of the histaminergic system during embryogenesis [6,7], postnatal development of nucleoli in histaminergic neurons [8]. To date, the role of the histaminergic system in the activity of the brain, participation in the regulation of systems and reactions of the body under normal conditions, as well as changes in its functional activity under various pathological conditions have been studied [2,9]. The localization, spatial organization, structure and features of the metabolism of histaminergic neurons of the hypothalamus in adult animals are described in the norm and under some experimental influences [1,2,10-12]. However, there is no information in the literature on the development of organelles of these neurons in the dynamics of postnatal development, which reflects the relevance of this study for neurobiology and developmental biology. The purpose of the study is evaluation of postnatal organellogenesis of E2 nucleus histaminergic neurons in the rat hypothalamus.
Materials and Methods
Animals, Chemicals and Experimental Design
The study was performed on the offspring of outbred white rats (12 pups), in accordance with the principles of bioethics and the requirements of the Directive of the European Parliament and of the Council No. 2010/63/EU of September 22, 2010, on the protection of animals used for scientific purposes [13]. This study received permission from the Biomedical Ethics Committee of the Grodno State Medical University (protocol No. 1 dated January 30, 2018). The animals were on a standard vivarium diet. Rats that reached the required age were taken out of the experiment by decapitation and the hypothalamus was taken. Decapitation of rat pups was carried out on the 5th, 20th, and 45th days after birth (for a better assessment of the dynamics of development, one rat pup was taken from each litter for each period, a total of 4 pups). Identification of brain structures was carried out according to the schemes of the stereotaxic atlas [14]. All the chemicals were obtained from Sigma- Aldrich (USA).
Electron Microscopy
For electron microscopy the lateral parts of posterior hypothalamus, where the histaminergic neurons of the largest group, E2, are situated, immediately after they were taken were placed in 1% osmium fixative in Millonig’s buffer (pH=7.4) [15] for 2 hours at a temperature of +4°C. The samples were washed in a mixture of Millonig’s buffer (20ml) and sucrose (900mg), dehydrated in 50° and 70° ethanol. The samples were kept for 12 hours in 70° ethanol. Then they were dehydrated in alcohols of increasing concentration, a mixture of alcohol and acetone, 3 portions of acetone, passed through a mixture of resin (Araldite M+ Araldite M hardener 964+dibutyl phthalate+ Araldite M accelerator 960,) and acetone, and then they were enclosed in resin in gelatin capsules and placed in a thermostat at a temperature of +60°C for 4 days for polymerization. Semithin sections 350nm thick were obtained with a ultramicrotome Leica EM UC 7 (Leica Microsystems GmbH, Germany) and stained with methylene blue. The preparations were examined under a light microscope to clarify the localization of E2 nucleus histaminergic neurons of the hypothalamus. For the identification of the E2 group of histaminergic neurons the stereotaxic atlas and corresponding topographic schemes were used [1,14].
Ultrathin sections (about 35nm thick) were made on a ultramicrotome Leica EM UC 7 (Leica Microsystems GmbH, Germany), collected on supporting copper grids (Sigma, cell size 300×83), and counterstained with uranyl acetate [16] for 20min. under a dark cover at room temperature, then washed in three portions of bidi stilled water for 25 seconds, counterstained with lead citrate for 8 minutes. [17] and washed in three portions of bidi stilled water for 30sec. The resulting preparations were examined with a transmission electron microscope JEM-1011 (JEOL, Japan). Ultra-photographs were acquired by digital camera (Olympus Mega View III, Germany) Ultrastructural morphometry was performed using the item image processing program (Version 5.0; Build 1224; Serial Number A3766900-7E852FAB, Germany), tracing selected objects on a computer monitor with the cursor and estimating their number and size.
Statistics
The primarily data obtained were treated by nonparametric statistics using software Statistical 10.0 (Stat Soft, Inc., USA). Quantitative results were presented as “Me (LQ; UQ)”, where Me is the median, LQ is the upper limit of the lower quartile, and UQ is the lower limit of the upper quartile. Comparison of groups on one basis was carried out using the Mann-Whitney U-test for independent samples. Differences between groups were considered statistically significant if the probability of an erroneous estimate did not exceed 5% (p<0.05).
Results
The results of an electron microscopic study showed that the ultrastructure of histaminergic neurons in the rat hypothalamus changes significantly in postnatal ontogenesis. In the cytoplasm of histaminergic neurons of 5-day-old rat pups, a moderate number of mitochondria is noted, which have a round, oval, oblong, occasionally irregular or branched shape and are located diffusely (Figure 1A, 2A). Sometimes their division is observed. Individual mitochondria are almost closely adjacent to the nuclear membrane, cisternae, and tubules of the endoplasmic reticulum or to each other, sometimes contacting each other. Mitochondrial cristae are not always well expressed and are arranged randomly: both along and across with respect to the long axis of the organelles. Sometimes they are bent and do not have a specific orientation (Figure 1A, 2A).
By the 20th day, mitochondria acquire a more elongated shape, which persists at later stages of development, and in terms of their localization, these structures do not differ significantly from those described earlier (Figure 1D). Branched organelles are observed more often than on the 5th day (Figure 1D, 2B). Dividing organelles are also found. On day 45, in the cytoplasm of histaminergic neurons, mitochondria are still in contact with other organelles and with each other (Figure 1G). Often, their local accumulations are noted near the nucleus or endoplasmic reticulum. There are branched organelles (Figure 2C). Dividing mitochondria are observed more often than in the previously described periods of postnatal development (Figure 1&2).
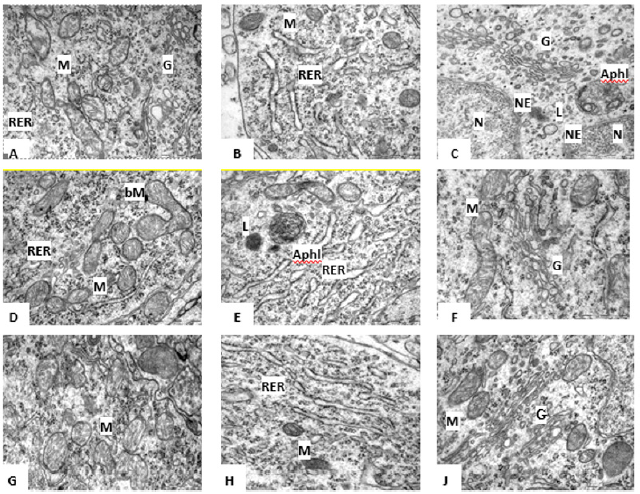
Figure 1:Mitochondria (A,D,G), Rough Endoplasmic Reticulum (B,D,H), Golgi Complex (C,F,I) in the cytoplasm of rat hypothalamus histaminergic neurons on the 5th (A,B,C), 2th (D,D,F) and 45th (F,H,I) days of postnatal development. Autophagolysosome (AL), Branched Mitochondria (bM), Golgi Complex (G), Lysosome (L), Mitochondria (M), Nuclear Envelope (NE), Nucleus (N), Rough Endoplasmic Reticulum (RER). Scale bar: 0.5 μm, ×50000 (A,D,G); scale bar: 0.5 μm, ×60000 (B, C, D, F, H, I).
From days 5 to 45 of postnatal development, the number of mitochondria in histaminergic neurons increases by 1.7 times: from days 5 to 20, as well as from days 20 to 45, by 1.3 times (p<0.001) (Table 1). At the same time, their individual area does not undergo significant changes (Table 1), while the change in the relative area of these organelles fully corresponds to the change in their number (Table 1). The length of mitochondrial cristae during the studied period of postnatal ontogenesis increases by 1.5 times: from the 5th to the 20th day-by 1.2 times (p<0.001), from the 20th to the 45th dayby 1.3 times (p<0.001) (Table 1).
The Rough Endoplasmic Reticulum (RER) in the histaminergic neurons of 5-day-old rat pups consists of numerous tubules and cisternae, various in length and tortuous, most often randomly scattered throughout the cytoplasm (Figure 1B). On the 20th day of postnatal development, RER is more pronounced, the number of cisternae and tubules increases in it, acquiring a more correct parallel orientation (Figure 1E). By the 45th day, the disordered arrangement of cisternae and tubules remains, the number of which increases (Figure 1H). At the same time, the length of the tubules and cisternae of the RER does not change significantly over the studied period (Table 1). The outer surface of the cisternae and tubules of the RER carries ribosomes, which are unevenly arranged, singly or in groups, lining up in a line; other parts of the membranes are free from ribosomes. From the 5th to the 45th day after birth, the number of bound ribosomes (per 1μm of the length of the tubules and cisternae of the RER) increases by 2.1 times: from the 5th to the 20th day-by 1.6times (p<0.001), from the 20th to the 45th day-by 1.3 times (p<0.001).
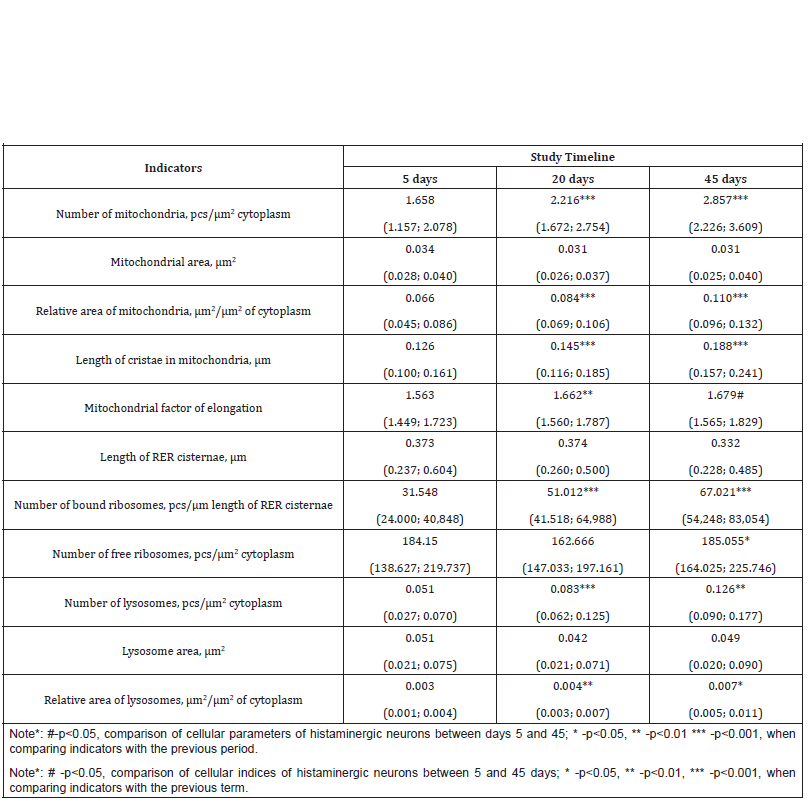
Table 1:Morphometric indicators of histaminergic neurons organelles in the period of postnatal development (Me (LQ; UQ)).
In addition to bound ribosomes in the cytoplasm of histaminergic neurons, there are many free ribosomes, lying both singly and combined into polysomes (on average, six pieces), which form rosettes and spirals. Their number decreases from the 5th to the 20th day (not statistically significant), and from the 20th to the 45th increases by 1.1 times (p=0.045). The Golgi complex on the 5th day is mainly represented by microbubbles, a few vacuoles, and single cisterns. It is near the nucleus (Figure 1C). As the animals grow older, the organization of the Golgi complex becomes more complex, manifested in an increase in the number of cisterns and their length. However, there are fragments of the Golgi complex, consisting of only two components-numerous small vesicles and larger vacuoles (Figure 1F). The Golgi complex on the 45th day is located exclusively in the perinuclear region and is detected in the form of several territorially separated zones, which may differ from each other both in the number and structure of cisterns, and in the number and size of bubbles (Figure 1J).
In the cytoplasm of histaminergic neurons on the 5th day there are single lysosomes, which are bodies of small, medium, and large sizes, filled with a homogeneous substance of uniform density, and with a heterogeneous content (Figure 3A, 3B). On the 20th and 45th days of postnatal development, lysosomes of various shapes are also present, the sizes of which do not change significantly, often, these organelles are near the Golgi complex (Figure 3C, 3D). In postnatal ontogenesis, the number of lysosomes increases by 2.5 times: from days 5 to 20, by 1.6 times (p<0.001), from days 20 to 45, by 1.5 times (p=0.004) (Table 1). At the same time, their individual area does not undergo significant changes (Table 1), and the relative area from the 5th to the 45th day increases by 2.3 times (from the 5th to the 20th day -1.3 times (p=0.005), from the 20th to the 45th day -1.8 times (p=0.033) (Table 1). Along with lysosomes in neurons for all the studied periods of development, multivesicular bodies are quite often found formations located mainly on the periphery of the cell, surrounded by a membrane and containing a different number of small vesicles with different osmiophilicity (Figure 2B) (Figure 3).
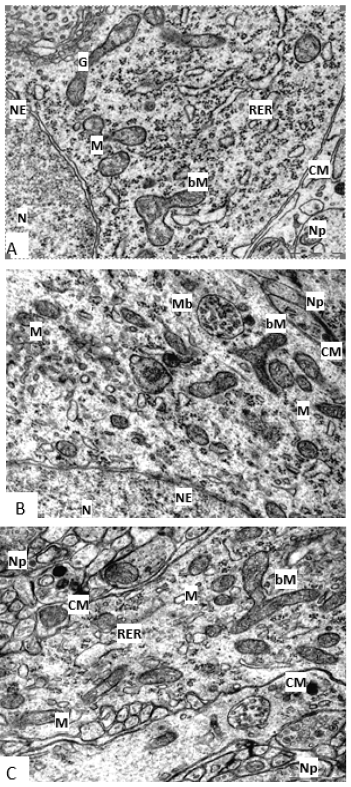
Figure 2:Branched mitochondria in rat hypothalamus histaminergic neurons on the 5th (A), 20th (B), 45th (C) days of postnatal ontogenesis.Branched Mitochondria (bM), Cell Membrane (CM), Golgi Complex (G), Mitochondria (M), Multivesicular Body (Mb), Neuropil (Np), Nuclear Envelope (NE), Nucleus (N), Rough Endoplasmic Reticulum (RER). Scale bar: 0.5 μm, ×30000.
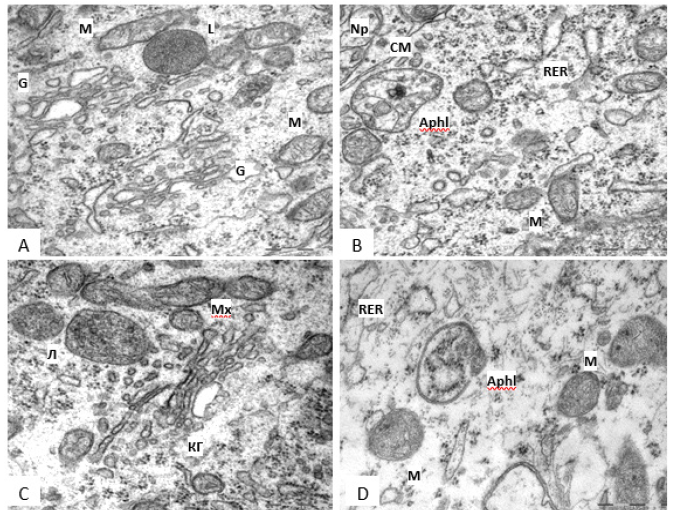
Figure 3:Variety of lysosomes in the cytoplasm of rat hypothalamus histaminergic neurons on the 5th (A,B) and 45th (C,D) days of postnatal development. Autophagolysosome (Aphl), Cell Membrane (CM), Golgi Complex (G), Lysosome (L), Mitochondria (M), Neuropil (Np), Emerging Autophagosome (Aph). Scale bar: 0.5 μm, ×60000.
Discussion
In postnatal ontogenesis in developing histaminergic neurons, the formation of functional cell apparatuses is traced: energy, synthetic, digestion and protection. Thus, as animals grow older in the studied neurons of the brain, the number of mitochondria and the relative area occupied by them in the cytoplasm increase, while they are elongated, and the length of the cristae increases in them. This reflects the formation of the energy apparatus of developing neurons. The observed contact of mitochondria with the nuclear membrane, cisterns and tubules of the RER indicates a high level of metabolic processes with significant energy costs in these zones [18]. It should be noted that in pyramidal neurons of the frontal cortex of the brain and Purkinje cells of the cerebellum in postnatal ontogenesis, elongation of mitochondria and an increase in the length of cristae were also observed in them; in addition, they also increased in size, but their number in Purkinje cells did not increase [19,20]. This indicates some features of the formation of the energy apparatus in the developing histaminergic neurons of the brain.
In developing histaminergic neurons from the 5th to the 45th day of postnatal development, branched forms of mitochondria are found. There is an opinion that in most cells’ mitochondria are not represented by isolated structures but are organized into a three-dimensional network of straight or branching chains of mitochondria or represent one or more branched organelles. Thus, what we call mitochondria, meaning a single organelle, may be only part of the “mitochondrial network”, which is a dynamic system that is modified depending on energy requirements and metabolic conditions, thereby indicating complexity, as well as functional and structural the integrity of the energy apparatus of the cell. Based on our data, we can assume the growth and branching of such a “mitochondrial network” in developing histaminergic neurons [21]. One of the important mechanisms of cellular adaptation is “mitochondrial dynamics”, which includes the process of mitochondrial division.
It has been established that not only ATP synthesis and oxidative stress reactions, but also processes such as cell growth and aging depend on the division of these organelles. The phenomenon of mitochondrial division has long been known. He was assigned the role of providing daughter cells with these organelles after mitosis. However, recently the division of mitochondria has been recognized as an important characteristic of not only dividing, but also interphase cells, including cells of such a highly differentiated tissue as nervous tissue. From the 5th to the 45th day of postnatal development of histaminergic neurons of the rat brain, dividing mitochondria are quite often found in their cytoplasm. The division of these organelles as one of the ways of mitochondrial biogenesis and an increase in the number of mitochondria provides the growing energy needs of the cell. Although this may also reflect the accelerated branching of the “mitochondrial network” mentioned above [21]. During the growth and differentiation of histaminergic neurons, the number of mitochondria and the relative area they occupy in the cytoplasm increase, while their average size does not undergo significant changes, which may be due to the active division of these organelles.
Thus, the division of mitochondria regulates their number and is a mechanism for controlling the renewal and quality of the mitochondrial population. The observed elongation of mitochondrial cristae in developing histaminergic neurons may reflect an increase in folding and, accordingly, the area of the inner membrane and an increase in the energy potential of mitochondria. Elongation of the cristae can lead to elongation of the mitochondria themselves, which is confirmed by an increase in the mitochondrial elongation factor from days 5 to 45 of postnatal ontogenesis in rats. As these neurons develop, the RER is formed. It increases the number of cisterns and tubules, the length of which does not change. At the same time, the ribosomes number on the RER membranes increases, which may be associated with an increase in the need for histaminergic neurons in protein biosynthesis for export, in the terminal. At the same time, the number of free ribosomes that provide protein biosynthesis for the needs of perikaryons per unit area of the cytoplasm does not change significantly in postnatal ontogenesis.
It should be noted that in the pyramidal neurons of the frontal cortex in the postnatal period, there is a significant increase in the number of bound ribosomes, but a decrease in the number of free ribosomes, although these indicators do not change significantly in Purkinje cells. However, in both types of cortical neurons, there is a significant progressive elongation of the RER cisternae [19,20]. The process of differentiation of histaminergic neurons of the hypothalamus in postnatal ontogenesis is accompanied by the formation of the Golgi complex, growth, and a more ordered arrangement of its cisterns, which was also observed in cortical neurons [19,20]. All the above reflects the general patterns and features of the development of the synthetic apparatus of histaminergic neurons of the brain in postnatal ontogenesis. In postnatal ontogenesis, the number of lysosomes in the cytoplasm of histaminergic neurons increases and, accordingly, an increase in their total relative area is observed. However, the size of lysosomes does not change significantly. It should be noted that in pyramidal neurons of the frontal cortex of the brain and Purkinje cells of the cerebellum, an increase in not only the number but also the size of lysosomes was observed [19,20].
This indicates the similarity and features of postnatal development in the histaminergic neurons of the brain of the intracellular digestion and protection apparatus necessary to ensure autophagy, remove worn out membranes and organelles damaged during the life process. It is noteworthy that histaminergic neurons on the fifth day of postnatal development, in comparison with pyramidal neurons of the cerebral cortex and Purkinje cells of the cerebellum, look morphologically more mature [20,22,23]. According to the literature data, the morpho functional differentiation of brain structures occurs in the caudal-rostral direction, starting from the spinal cord and medulla oblongata and ending with the cerebral cortex and cerebellum. Thus, phylogenetically older cells of the underlying brain regions differentiate earlier than cells of the overlying regions. The formation of the ultrastructure characteristic of mature neurons, first, is completed in the structures of the brain stem and later in the cerebral cortex. In this case, the order of differentiation reflects the sequence of functional maturation of cells. This lack of simultaneous maturation of neurons in different brain structures enables them to function in accordance with their significance at each stage of development. According to the literature data, monoaminergic systems are among the earliest developing neurotransmitter systems in the mammalian brain [24,25].
Thus, the differentiation of histaminergic neurons begins even in embryogenesis. On the 16th day of prenatal development, the enzyme of histamine synthesis, histidine decarboxylase, is detected in them for the first time. Until birth, there is a migration of GDCimmunoreactive neurons, as a result of which they occupy a location in the hypothalamus characteristic of histaminergic neurons in adult animals. In this case, the neurons of the tuberomammillary region undergo their final mitotic division before the expression of the mediator that determines their phenotype [26,27]. By the 5th day after birth, the histaminergic system of rat pups is anatomically like the histaminergic system of an adult animal, since even in embryogenesis, histaminergic neurons migrate to their final location, where they form groups of E1-E5 nuclei, stop dividing, and form processes [7,26,27]. At the same time, the formation of the cortical structures of the brain through the migration of neurons to the place of their final localization from the areas of their “birth” is completed only in postnatal ontogenesis [28]. However, in structural and functional respects, histaminergic neurons in early postnatal ontogenesis show signs of immaturity. Then, during postnatal development, they show signs of differentiation like other types of brain neurons, reflecting the general patterns of their postnatal ontogenesis.
Conclusion
In the postnatal ontogenesis of the rat a regular development of organelles of histaminergic neurons of the hypothalamus is observed. Thus, the number of mitochondria and the relative area occupied by them in the cytoplasm increase in them, but their average size does not change, the mitochondria are elongated, and the length of the cristae increases in them. At the same time the number of bound ribosomes on the RER cisternae increases, while the number of free ribosomes does not change, the Golgi complex develops progressively and the number of lysosomes increases, but not their size. This reflects the patterns and features of the postnatal development of the functional apparatuses of histaminergic neurons of the brain: energy, synthetic, digestion and protection.
Author Contributions
All authors made the same contribution to this study and the preparation of the article.
Conformity with the Principles of Ethics
The study was performed in accordance with the principles of bioethics and the requirements of the Directive of the European Parliament and of the Council No. 2010/63/EU of September 22, 2010, on the protection of animals used for scientific purposes.
Acknowledgement
This work was supported by the Belarusian Republican Foundation for Basic Research (M20M-089).
Conflict of Interest
Authors declare that they have no financial or personal conflicts of interest that could inappropriately influence the conduct of this research.
References
- Dalziel JM (1937) The Useful Plants of West Tropical Africa. Crown Agents for the Colonies, London.
- Iwu MM (1993) Handbook of African medicinal Plants. Boca Raton, CRC Press Inc: pp. 223-224.
- Adesina SK, Gbile ZO, Odukoya OA, Akinwusi DD, Illoh HC, et al. (1995) Survey of indigenous plants of West Africa with special emphasis on medicinal plants and issues associated with management. The United Nations Programme on Natural Resources in Africa 2nd (Edn) 84-85.
- Ayensu ES (1978) Medicinal Plants of West Africa. Refer Publ Inc Algonac Mich pp. 162.
- Ajebesone PE, Aina JO (2004) Potential African Substitutes for Hops in Tropical Beer Brewing. J Food Tech in Africa 9(1): 13-16.
- Nester EW, Roberts LE, Pearsall NN, Anderson DG, Nester MT, et al. (2004) Antimicrobial medicine: In Keven T. K., Ronald., E. N., Terrace, S., Jode, K. B. (eds). Microbiology A human perspective 2nd (Edn), Von Hoffman press Inc, University of Washington, New York. pp 447-460.
- Okunji CO, Iwu MM (1991) Molluscidal activity of Garcinia kola biflavonones Fitoterapia 67: 74-76.
- Terashima K, Kondo Y, Aqil M, Waziri M (1999) A study of biflavanones from the stem of Garcinia kola. Heterocycles 50: 238-290.
- Iwu MM (1985) Antihepatotoxic constituents of Garcinia kola seeds and stem. Experientia 41(5): 699-700.
- Trease GE, Evans WC (1983) Pharmacognosy. 14th (Edn), Publ Brown Publications.
- Cheesbrough M (2000) summary of the clinical and laboratory features of microorganisms in: District laboratory practice in Tropical countries. Cheesbrough M (Edn) Part 2, Cambridge university press pp. 178-244.
- Cowan ST, Steel KJ, (1965) Antibiotic sensitivity In: Cowan Steel’s manual for identification. Cambridge university press, London, New York. pp. 24.
- Ebana RU, Madunagu BE, Ekpe ED, Otung IN (1991) Microbiological exploitation of cardiac glycosides and alkaloids from Garcinia kola, Borreria ocymoides, Kola nitida and Citrus aurantifolia. J Appl Bacteriol 71(5): 398-401.
- Iwu MM, Igboko OA, Okunji CO, Tempesta MS (1990) Antidiabetic and aldose reductase activities of biflavones of Garcinia kola. J Pharm Pharmacol 42(4): 290-292.
- Okunji CO, Tantalia AW, Hicks RP, Iwu MM, Skanchy DJ, et al. (2002) Capillary electrophoresis determination of biflavonones from Garcinia kola in three traditional African medicinal formulations. Plant Med 68(5): 440-444.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.